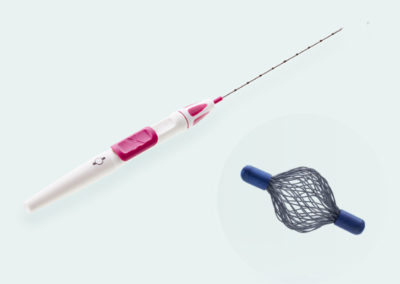
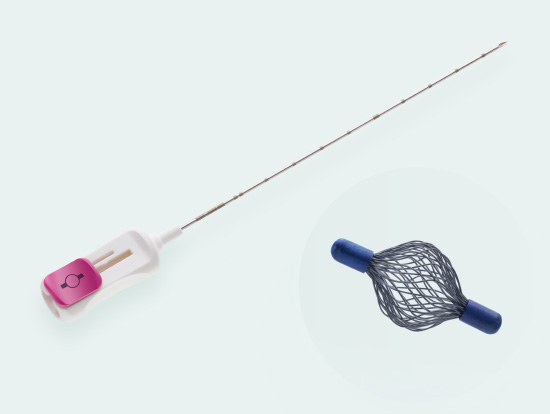
Tumark® Vision
Tried and tested 3D marker with hyperechoic structure

Visibility
Intelligent 3D design of the vision-marker provides very good visibility in ultrasound, X-ray and MRI

Stability
Marker expands into shape upon deployment and anchors firmly in the tissue

Single-handed operation
Ergonomic handle allows for single-handed use
Tumark® Vision
Spherical premium marker
Tumark Vision is our spherical 3D marker for marking biopsy sites and suspicious lesions in the breast tissue. The marker is designed to provide improved ultra- sound imaging. Its mesh structure in a three-dimensional shape has high echogenicity regardless of transducer positions. In other imaging procedures (X-ray, MRI), the spherical marker shape is also very well detectab
Long-term stability
The long-term visibility of a biopsy site marker is particularly important in neo- adjuvant chemotherapy (NACT). The Tumark Vision tissue marker expands upon deployment and anchors fi rmly in the tissue. It is made from the biocompatible implant material Nitinol and remains visible
- Unique 3D design with a mesh structure made of 48 individual wires leads to high echogenicity
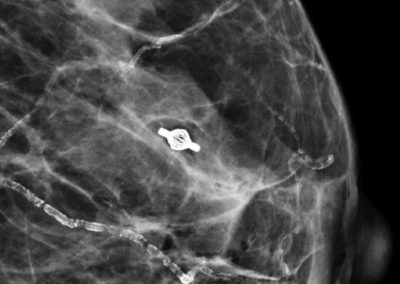
- Very good visibility in ultrasound, X-ray and MRI [1]
- Long-term stability, particularly important in neoadjuvant chemotherapy (NACT)
- 18 Gauge thin, sharp puncture cannula
- Cannula with cm-markings for depth orientation
- Slide button on handle for marker deployment
- Ergonomic handle allows for single-handed use
Sharp beveled tip with ultra-sonically visible marking
Tumark Vision site marker is MRI-compatible. Tumark Vision application device is not suitable for use in MRI. For MRI-guided marking, we recommend Tumark Vision MRI.

The videos were filmed in the breast center of Evang. Kliniken Essen-Mitte, in Essen, Germany and show the everyday clinical practice there.
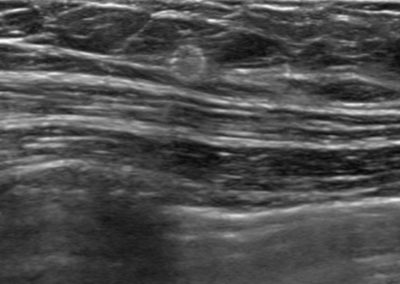
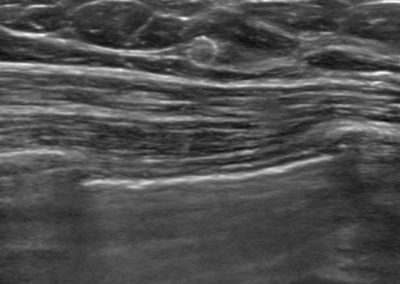
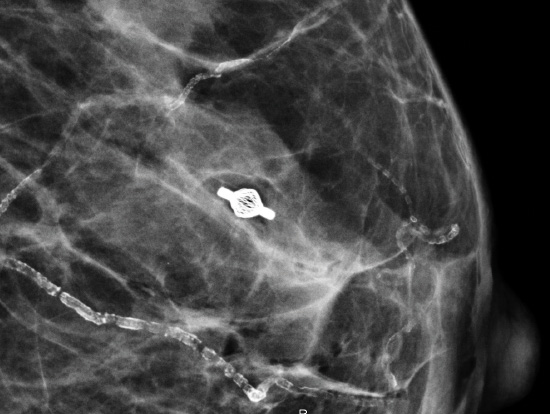
Tumark® Vision in imaging methods
Tumark Vision in ultrasound, 3 months after marking with tumor findings not (no longer) clearly definable
Tumark Vision in ultrasound, 5 months after marking following completion of NACT (6xTCbHP) with tumor findings not (no longer) clearly definable
Tumark® Vision Study2
Our products regularly prove themselves in independent studies. A study conducted by the clinics of Essen Mitte in Essen and the Charité in Berlin, Germany rates the clinical application of Tumark Vision as excellent. In 50 patients at the Essen Mitte Clinics, all 50 primary lesions as well as 23 axillary lymph nodes were marked with the Tumark Vision marker[3].
2Source: Rüland, A. M., Hagemann, F., Reinisch, M., Holtschmidt, J., Kuemmel, A., Dittmer-Grabowski, C., Stöblen F., Rotthaus H., Dreesmann V., Blohmer J.-U. Kuemmel, S. (2018). Using a New Marker Clip System in Breast Cancer: Tumark Vision Clip-Feasibility Testing in Everyday Clinical Practice. Breast Care, 13(2).
3Marking lymph nodes with Tumark Vision is not CE-approved (October 2021).
Visibility of Tumark® Vision on the day of application
- Very good 72%
- Good 24%
- Poor 4%
- 100% stability
In all cases, the marker remained firmly anchored in the tissue. - 100% visibility
In all cases, the marker could be identified under ultrasound. - 100% handling
In all cases, the application of the marker was unproblematic.
Tumark® Vision MRI
MRI version for MRI-guided breast tissue markin
Tumark Vision MRI is constructed for MRI-guided site marking up to three Tesla. The compact design of the MRI-compatible deployment device is suitable for use in the MRI gantry.
Order information
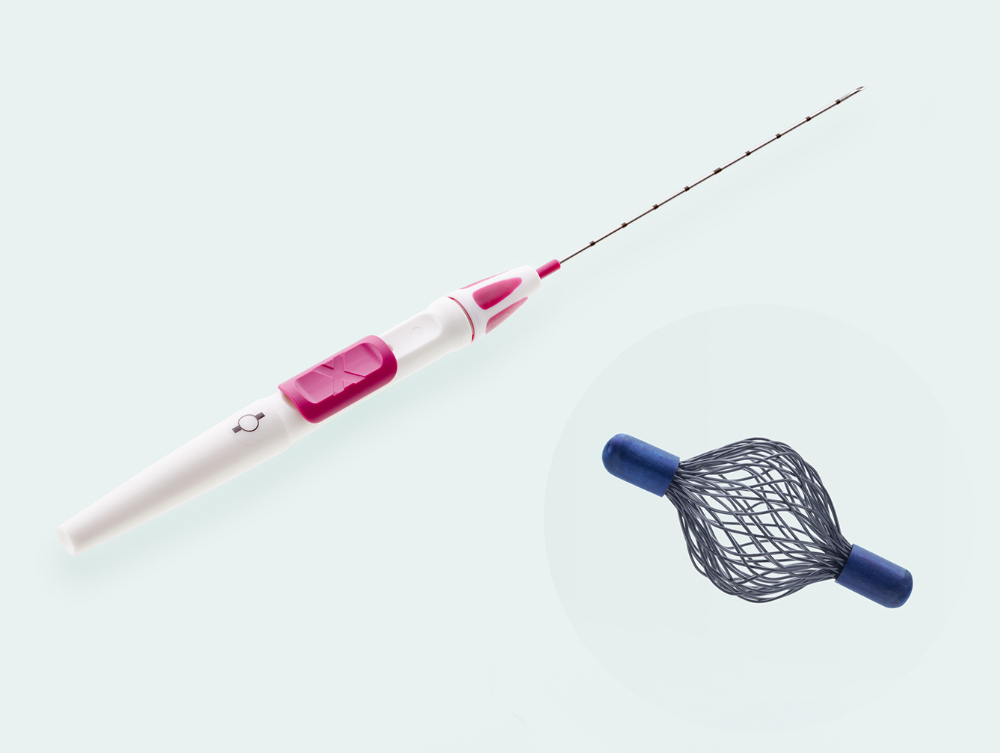
Tumark® Vision
271589 / 18 G / 100 mm
271590 / 18 G / 120 mm

Tumark® Vision MRI
601590 / 18 G / 120 mm
Packing unit per product: 10 pieces Shelf life from production date: 5 years
Tumark® Vision Atlas
Case studies
The Tumark Vision Atlas contains case studies of the Tumark Vision biopsy site marker and provides clinical information. The long-term visibility of a biopsy site marker is particularly important in neoadjuvant chemotherapy. Some of the following case studies report this application scenario.